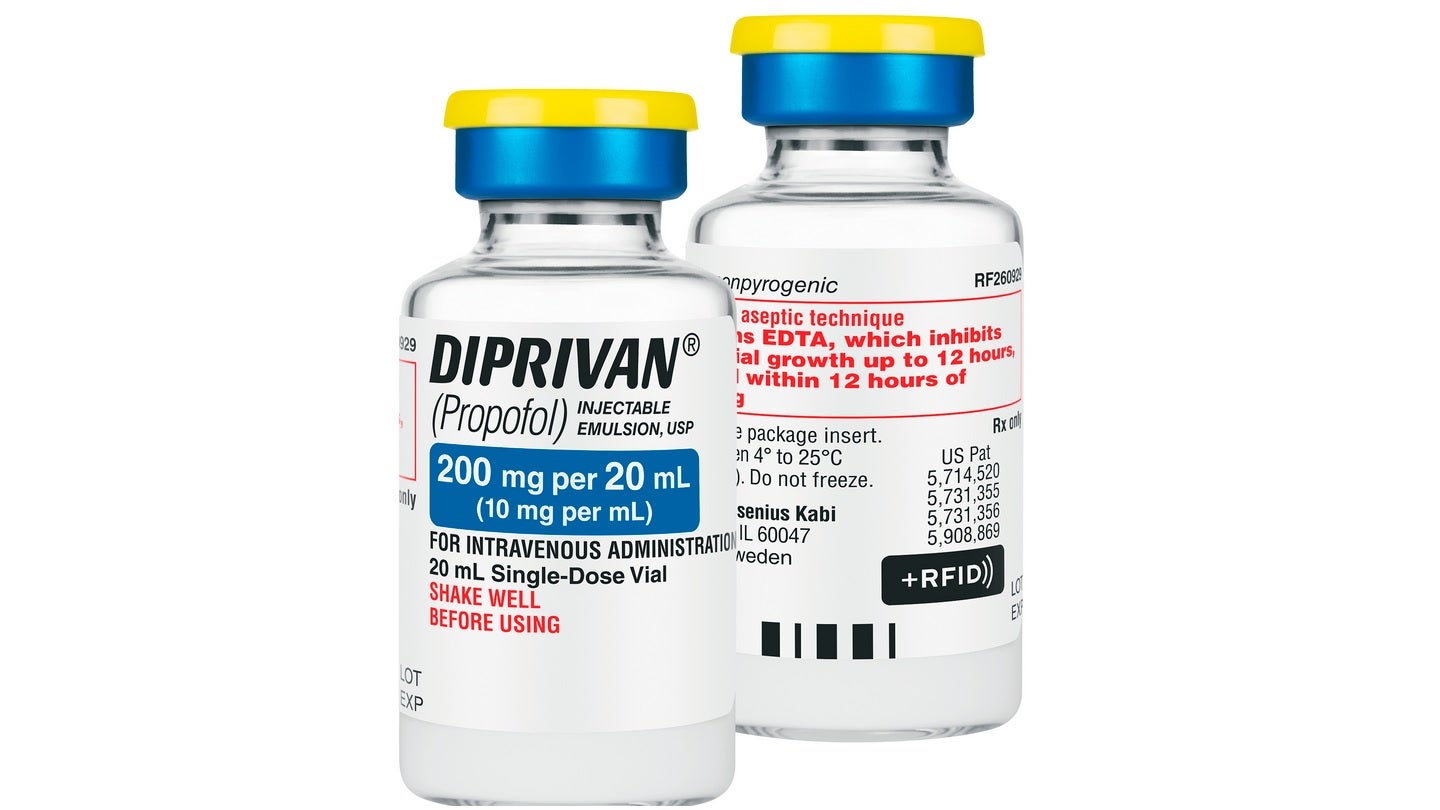
Fresenius Kabi has announced the launch of +RFID smart labels for Diprivan (Propofol) injectable emulsion, USP, 200mg per 20ml in single-dose vials, in the US market.
These smart labels are compatible with all ‘major’ radio-frequency identification (RFID) kits and tray systems in the country.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This RFID label technology removes the manual tagging of medicines, thereby saving time and enhancing the safety and efficiency of the medication inventory management process.
Aside from Fresenius’ testing, Diprivan +RFID has been tested by The Axia Institute of Michigan State University and certified by the ARC RFID laboratory of Auburn University to ensure it meets national performance and quality standards.
The company is extending its fully compatible +RFID product portfolio in response to hospitals’ growing demands across the country.
Fresenius claims to be the first pharmaceutical manufacturer to take up GS1’s EPC Tag Data Standard for enhancing interoperability.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFresenius USA president and CEO John Ducker said: “We are dedicated to supporting our customers in the adoption of RFID technology that enhances patient care, improves efficiency and streamlines workflows for pharmacists.”
In 2020, Diprivan became the first product to feature RFID labels from Fresenius for use with specific RFID-compatible kits and tray systems.





