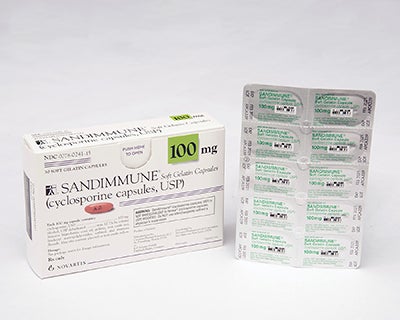
Swiss pharmaceutical giant Novartis has recalled some of its Sandimmune and Neoral blister packs in the US over a packaging issue.
According to the company, the recall will affect Sandimmune (cyclosporine capsules, USP) 100mg soft gelatin capsules 30-count pack and liquid-filled single capsule blister packs.
It also covers Neoral (cyclosporine capsules, USP) MODIFIED 100mg soft gelatin capsules 30 count pack and liquid-filled single capsule blister packs.
The company identified that the 30-count blister packs distributed in the US failed to meet child-resistant packaging guidelines.
In response to this, the company issued a Consumer Product Safety Commission (CPSC) approved corrective action plan.
The packaging problem could pose harm if a child opened the pack and swallowed the medicine. Patients have been advised to keep the packs out of children’s reach to ensure safety.
In a statement, Novartis said: “At Novartis, we take our responsibility for consumer safety very seriously.
“As soon as we became aware that these blister packs do not meet federal standards for child-resistant packaging, we promptly notified the CPSC and the Food and Drug Administration (FDA) and have been working closely with the CPSC to remedy the packaging issue and ensure continuity of treatment.
“An appropriate solution was identified in cooperation with the CPSC.
“There are no quality or efficacy issues with the medicines for their intended use.”
To avoid shortage of the drug, the company is providing resealable child-resistant pouches to those affected.



