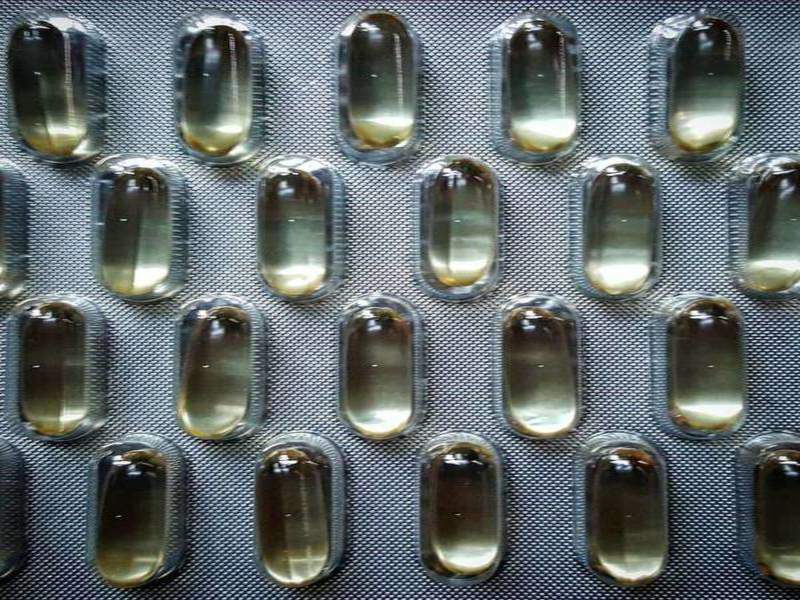
Tekni-Films has developed a new film for pharmaceutical blister packs that will become part of the company’s portfolio of super barrier-coated (SBC) thermoformable film.
The SBC 240 film serves as an alternative for 4-mil and 6-mil polychlorotrifluoroethylene (PCTFE) and cold-formed foil in thermoformable blister applications, offering benefits in terms of improved performance and costs.
The triplex structure of the film makes it suitable for applications such as pharmaceuticals, nutraceuticals, probiotics and other related products.
SBC 240 does not require stiffening ribs, therefore improves the ability of PCTFE to lie flat, as well as the oversized blister wells created by cold forming.
The film will allow pharmaceutical companies to user smaller blister cards to contain the same number of tablets or capsules, as well as increase such number.
This will reportedly lead to considerable savings in the costs of the packaging process, and to improved production efficiencies.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe moisture and oxygen barrier properties offered by SBC 240 enable protection of products prone to degradation.
The wider processing window of the film also helps meet specific production speed preferences.
Tekni-Films has developed SBC 240 by applying a high-barrier variant of polyvinylidene chloride (PVdC) coating to a film structure prepared from layers of polyethylene.
The company’s offering also includes 120g, 150g, 180g and 210g coating thicknesses, as well as other custom solutions.
The company supplies flexible films, lidding foils and pouch materials for pharmaceutical, medical, diagnostic health and personal care products.



