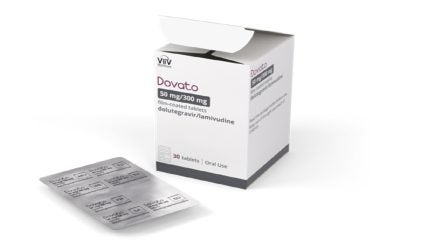
ViiV Healthcare, a global company specialising in human immunodeficiency virus (HIV), has announced the availability of Dovato in a new blister pack format in the US.
Dovato is a once-daily, single-pill, two-drug prescription regimen to treat HIV-1 in adults.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dovato’s blister pack aims to offer a discreet and convenient medication management option for people with HIV.
According to the company, the packaging was developed in response to feedback from those with HIV, addressing concerns such as prejudice and the need for greater privacy.
The newly approved blister pack contains a 30-day supply of medication, with each box holding five sheets of tablets.
The sheets are perforated, allowing individuals to track their dosage and remaining pills.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe US Food and Drug Administration (FDA) has already approved the blister pack and the company is planning to introduce the packaging to select European markets this year.
ViiV North America head Lynn Baxter said: “The Dovato blister pack is designed to help address some of the challenges we hear from the HIV community, which include stigma and convenience, and offers a discreet package which may fit more seamlessly into people’s daily routines.
“Everyone has different experiences and preferences when it comes to their HIV treatment and, at ViiV Healthcare, we are pleased to offer a variety of treatment and packaging options that help suit the needs of people living with HIV.”
The company will continue to provide Dovato in the traditional 30-count pill bottle format.





