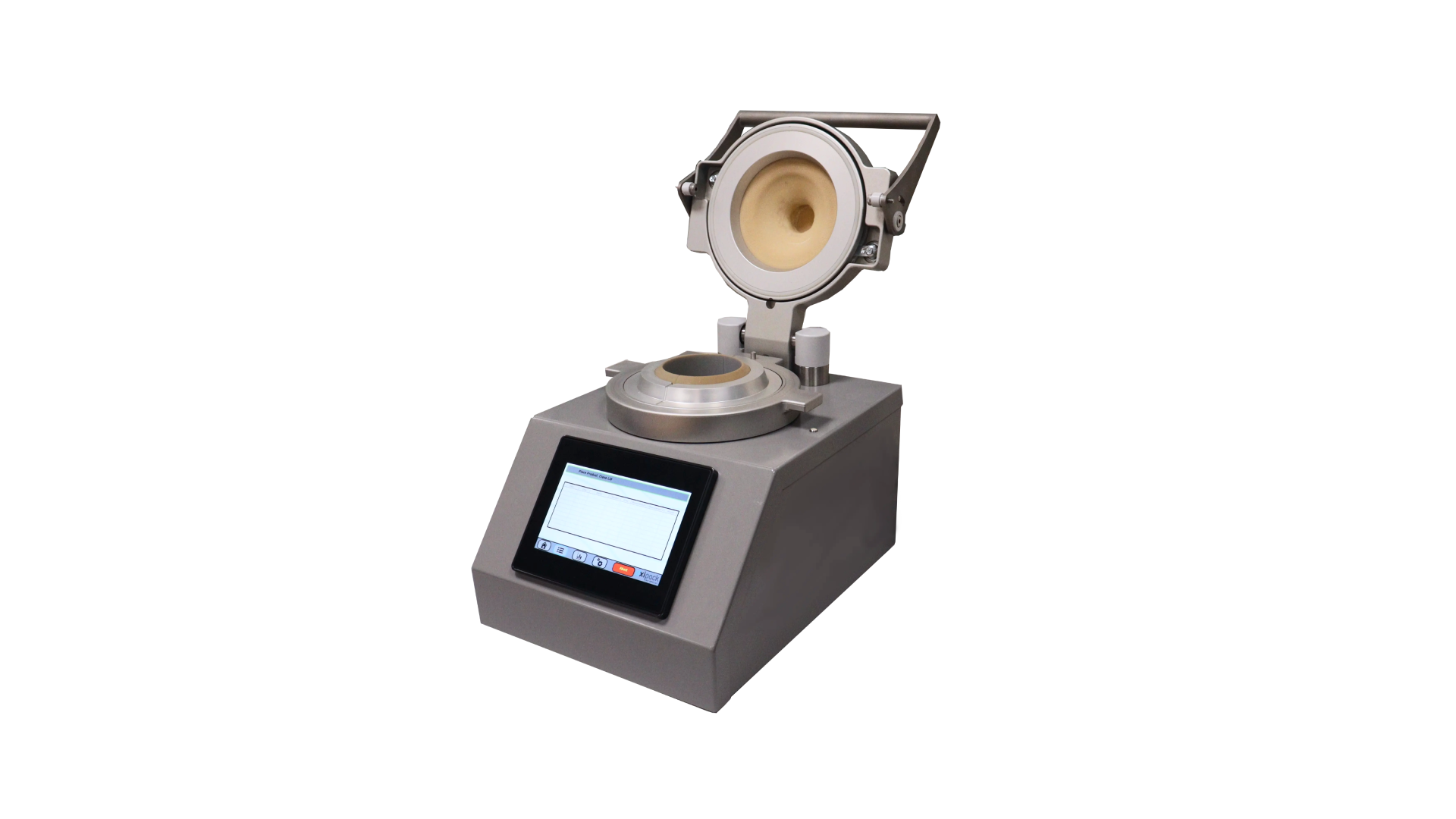
The Container Closure Integrity Tester (CCIT) offers a state-of-the-art solution for high-precision leak detection in the pharmaceutical and biological industries. This machine ensures product quality by testing the airtight seals of vials, ampoules, and bottles using the vacuum decay method (ASTM F2338). The CCIT is ideal for companies that require non-destructive leak testing to comply with stringent regulatory standards, such as USP 1207, which governs container closure systems in pharmaceuticals.
Key Features of the CCIT
- Non-Destructive Testing: The CCIT uses a flexible, FDA-approved membrane to detect leaks without damaging the container or its contents. This non-destructive approach allows tested products to be reintroduced into the production line if they pass the integrity test, minimizing waste and cost.
- Adaptability to Various Container Types: The CCIT is designed to accommodate a wide range of container types, including vials, ampoules, and bottles, without the need to change the test chamber. This feature speeds up the testing process and improves overall production efficiency.
- Precision in Leak Detection: The CCIT can detect micro-leaks as small as 0.035cm³/min, ensuring the highest level of accuracy. This makes it ideal for sensitive pharmaceutical products where even the smallest breach can lead to contamination or product degradation.
- User-Friendly Interface: The machine features a touchscreen display that shows real-time test results, making it easy for operators to manage and monitor the testing process. The interface allows for easy adjustment of settings to accommodate different testing requirements.
Technical Specifications
- Dimensions and Weight: 400 x 300 x 350 mm (L x W x H); 20 kg
- Materials: Stainless steel, anodised aluminium, polycarbonate, rubber (Accura Xtreme)
- Power Supply: 230V 50/60HZ
- Air Supply: > 5.5 – < 8 bar
- Compliance: CE IP20
- Leak Detection Method: Vacuum decay (ASTM F2338)
- Minimum Leakage Detection: < 0,035 cm3/min
- Size Measuring Chamber: Up to 500 ml Vials
- Connections: USB/Ethernet export, 24VDC logic (free programmable)
- Supported Packaging Types: Vials, ampoules, and bottles.
Applications in Pharmaceuticals
The CCIT is particularly valuable for:
- Pharmaceuticals: Ensuring the quality of containers storing sterile drugs and preventing contamination.
- Biotechnology: Maintaining the quality of biologics and vaccines by preventing exposure to external contaminants.
Compliance and Regulatory Alignment
The CCIT complies with USP 1207 guidelines, which define methods for ensuring container closure integrity in pharmaceutical products. Adhering to these standards ensures product safety, patient health, and regulatory compliance. By implementing the CCIT, manufacturers can confidently meet the requirements of agencies such as the FDA and EMA.
Sustainability and Cost Efficiency
The CCIT contributes to sustainable production processes by reducing material waste through its non-destructive leak detection. Containers that pass the test can be reused, saving both costs and resources while maintaining the highest standards of product integrity.
The CCIT is the ultimate solution for businesses looking for a precise, efficient, and sustainable way to ensure the integrity of pharmaceutical and biological packaging. With its advanced vacuum decay technology, real-time feedback, and non-destructive testing capabilities, the CCIT helps pharmaceutical companies ensure the safety and efficacy of their products.


